UPDATE August 11, 2022: Boehringer Laboratories now offers our own solution for external urinary management for women, CareDry®. The CareDry® device is designed for liquid collection regardless of patient position or canister placement. Learn More Here
When using female external catheters (FECs) such as PureWick® and PrimaFit®, the location of the suction canister is a crucial and often overlooked aspect of the clinical setup.
Think of your suction regulator’s pressure setting as horsepower. If you need to suction urine downward, you don’t need much power, as gravity helps transport the urine. You’ll find if you have the suction canister placed on the floor, your PureWick® is much less likely to leak and will operate very well at low levels of pressure. Low pressure means better safety for your patients.
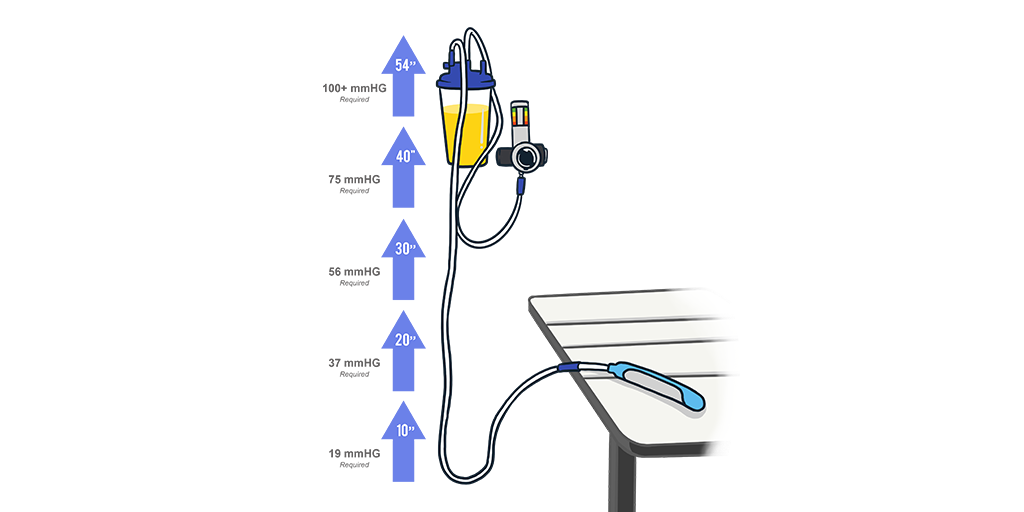
Unfortunately, placing canisters on the floor is not a very practical strategy as they are prone to get knocked over. Even worse, suction canisters often are mounted to the hospital’s wall, well above the location of the PureWick® or PrimaFit® external female catheters. In this situation, gravity works against you because you are essentially trying to pull the fluid “uphill.” If you’ve ever seen a semi-truck driving up a hill, this has the same effect, and you’ll notice they require a lot of power and don’t move very fast. As a rough rule of thumb, every foot of height takes 22mmHg. So if you set a regulator to 40mmHg, you won’t even be able to get fluid to rise two feet.
To compensate for the hardcore realities of gravity and physics, clinicians generally increase the suction. Turning the pressure higher on a suction regulator is the equivalent of a driver pressing harder on the gas pedal. Unfortunately, you can only increase the regulator’s pressure so much before you begin putting your patient at risk of suction-related injuries. While there is no hard-and-fast rule on what suction pressure is too high for PureWick® and other FECs, Boehringer Laboratories doesn’t recommend turning the pressure to greater than 65mmHg.
If you struggle with leakage or suction being set too high, look at your suction canister placement. A simple change in the location of your canister could make a big difference in the effectiveness of your PureWick® or PrimaFit® female external catheters.
Looking for more information on solutions for clinical suction? Be sure to check out Boehringer Suction Regulators.
PureWick® is a registered trademark of C.R. Bard, Inc.
PrimaFit® is a registered trademark of Sage Products, LLC
Back to Blog